Travel Medicine Centre
Yellow Fever &
Diseases Subject to International
Health Regulations
Yellow Fever 
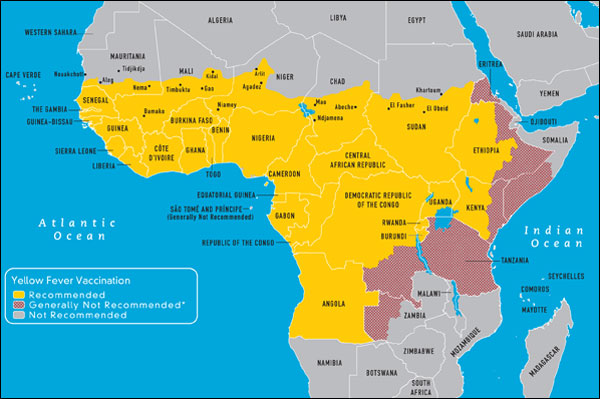
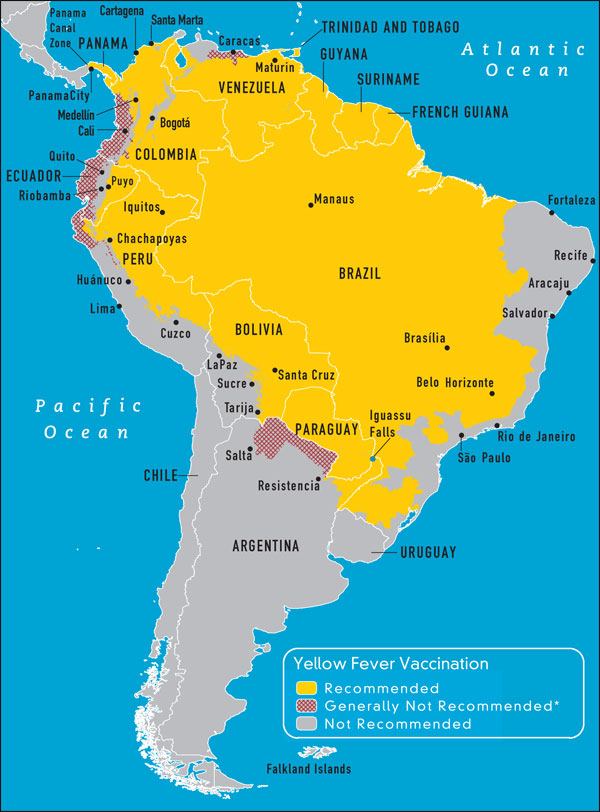
Yellow fever inoculation is recommended to all travelers in transit or going to the yellow fever endemic zones of Africa and Central and South America. In many cases it is required. There are no yellow fever endemic areas in Asia and travelers to these areas do not require yellow fever unless they go through Africa or South America. Yellow fever vaccine must be administered in a WHO approved vaccination center. Yellow fever is a live vaccine grown in eggs. It should not be given to those with cancer or to those with decreased immunity. It should not be given during pregnancy unless the risk of yellow fever is very high. It should be avoided in patients with a known allergy to eggs. Intradermal skin testing can be done to determine sensitivity in these cases. The vaccine is given to those over 6 to 9 months of age and after a single dose the certificate is valid for 10 years starting 10 days after administration.
As of June 2016 yellow fever vaccination certificates will be valid for life. Those travelers with contraindications to use or infants under 9 months of age should be given a certificate of exemption.
Yellow Fever Endemic zones - Africa (WHO CDC)
Yellow Fever Endemic zones - Americas (WHO CDC)
More info onYellow Fever Vaccine from CDC
Yellow fever is preventable by a relatively safe, effective vaccine. All yellow fever vaccines currently manufactured are live attenuated viral vaccines.
YF-VAX, the only yellow fever vaccine approved for use in the United States and Canada, is manufactured by
sanofi pasteur.
Studies comparing the reactogenicity and immunogenicity of various yellow fever vaccines,
including those manufactured outside of the United States, suggest that there is no significant difference in
the reactogenicity or immune response generated by the various vaccines. Thus, individuals who receive yellow
fever vaccines in other countries should be considered protected against yellow fever.
Recommendations for the Use of Yellow Fever Vaccine for Travelers
Persons aged ≥9 months of age who are traveling to or living in areas with risk of yellow fever transmission in South America and Africa should be vaccinated. In addition, some countries require proof of yellow fever vaccination for entry. See the following section in this chapter (Yellow Fever Vaccine Requirements and Recommendations, by Country) for more detailed information on the requirements and recommendations for yellow fever vaccination for specific countries.
However, because severe adverse events (see below) can follow yellow fever vaccination, physicians should be
careful to administer the vaccine only to persons truly at risk of exposure to YFV.
Refer to
Yellow Fever
Vaccine Recommendations of the Advisory Committee on Immunization Practices (ACIP)
for additional information or
Vaccine Dose and Administration
For all eligible persons, a single injection of 0.5 mL of reconstituted vaccine should be administered subcutaneously.
The International Health Regulations (IHR) published by WHO require revaccination at 10-year intervals.
Vaccine Safety and Adverse Reactions
Common Adverse Events
Reactions to yellow fever vaccine are generally mild, with 10%–30% of vaccinees reporting mild systemic adverse events.
Reported events typically include low-grade fever, headache, and myalgias that begin within days after vaccination and last 5–10 days.
Approximately 1% of vaccinees temporarily curtail their regular activities because of these reactions.
Severe Adverse Events
Hypersensitivity
Immediate hypersensitivity reactions, characterized by rash, urticaria, or asthma or a combination of these, are uncommon. Anaphylaxis following yellow fever vaccine is reported to occur at a rate of 1.8 cases per 100,000 doses administered.
Yellow Fever Vaccine-Associated Neurologic Disease (YEL-AND)
YEL-AND represents a conglomerate of different clinical syndromes, including meningoencephalitis, Guillain–Barré
syndrome (GBS), acute disseminated encephalomyelitis (ADEM), bulbar palsy, and Bell’s palsy.
Historically,
YEL-AND was seen primarily among infants as encephalitis, but more recent reports have been among persons of
all ages.
The onset of illness for documented cases ranges 3–28 days after vaccination, and almost all
cases were in first-time vaccine recipients.
• YEL-AND is rarely fatal.
The incidence of YEL-AND in
the United States is 0.8 per 100,000 doses administered. The rate is higher in persons ≥60 years of age, with
a rate of 1.6 per 100,000 doses in persons 60–69 years of age and 2.3 per 100,000 doses in persons ≥70 years
of age.
Yellow Fever Vaccine-Associated Viscerotropic Disease (YEL-AVD)
YEL-AVD is a severe illness similar to wild-type disease, with vaccine virus proliferating in multiple organs and often leading to multisystem organ failure and death.
Since the initial cases of YEL-AVD were published in 2001, more than 40 confirmed and suspected cases have been reported throughout the world.
The onset of illness for YEL-AVD cases averaged 3.5 days (range: 1–8 days) after vaccination. YEL-AVD appears to occur after the first dose of yellow fever vaccine rather than with booster doses.
The case–fatality ratio for reported YEL-AVD cases is 53%.
The incidence of YEL-AVD in the United States is 0.4 cases per 100,000 doses of vaccine administered. The rate is higher for persons ≥60 years of age, with a rate of 1 per 100,000 doses in persons 60–69 years of age and 2.3 per 100,000 doses in persons aged ≥70 years of age.
Risk of Dying from common risks (per year)
recent outbreaks WHO
Cholera 
The existing injectable cholera vaccines do not prevent the development of the carrier state or affect the severity of the disease. They do not prevent the introduction of cholera into a country or interrupt transmission. The risk to travelers is low. The International Health Regulations abolished the requirement of a vaccination certificate in June 1973. No country should require a certificate from travelers arriving from Canada. We provide an exemption certificate to each traveler which is sufficient to avoid inconvenience. A new oral cholera vaccine is available in some countries and is relatively effective for those few travellers at high risk.
Dukoral 
There is a new oral Cholera. A few such
as aid workers or those going to very remote areas with cholera outbreaks may want it to prevent cholera. Cost
$40 per dose 2 doses required (plus boosters for frequent travellers).
Health Canada/CATMAT does NOT recommend this vacine to
prevent travellers diarrhea
link see vaccine
Smallpox 
Effective January 1982, smallpox was eliminated as a quarantinable disease. No country requires a vaccination certificate and distribution of the vaccine for civilian use was discontinued in May 1983. The last reported case was 1977.
 Travel Medicine Center
Travel Medicine Center